Public Health Statement for Ionizing Radiation
Spanish: Radiación Ionizante
PDF Versionpdf icon[298 KB]
This Public Health Statement is the summary chapter from the Toxicological Profile for ionizing radiation. It is one in a series of Public Health Statements about hazardous substances and their health effects. A shorter version, the ToxFAQsTM,
is also available. This information is important because this substance may harm you. The effects of exposure to any hazardous substance depend on the dose, the duration, how you are exposed, personal traits and habits, and whether other chemicals are present. For more information, call the ATSDR Information Center at 1-800-232-4636.
This public health statement tells you about ionizing radiation and the effects of exposure. It does not tell you about non-ionizing radiation, such as microwaves, ultrasound, or ultraviolet radiation.
Exposure to ionizing radiation can come from many sources. You can learn when and where you may be exposed to sources of ionizing radiation in the exposure section below. One source of exposure is from hazardous waste sites that contain radioactive waste. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites make up the National Priorities List (NPL) and are the sites targeted for federal cleanup. However, it's unknown how many of the 1,467 current or former NPL sites have been evaluated for the presence of ionizing radiation sources. As more sites are evaluated, the sites with ionizing radiation may increase. This information is important because exposure to ionizing radiation may harm you and because these sites may be sources of exposure.
When a substance is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. This release does not always lead to exposure. Even in the event that you are exposed, it does not necessarily mean you will be harmed or suffer longterm health effects from exposure to ionizing radiation.
If you are exposed to ionizing radiation, many factors determine whether you'll be harmed. These factors include the dose (how much), the duration (how long), and the type of radiation. You must also consider the chemicals you're exposed to and your age, sex, diet, family traits, lifestyle, and state of health.
What is ionizing radiation?
To explain what ionizing radiation is, we will start with a discussion of atoms, how they come to be radioactive, and how they give off ionizing radiation. Then, we will explain where radiation comes from. Finally, we will describe the more important types of radiation to which you may be exposed. Of the different types and sources of ionizing radiation, this profile will discuss the three main types: alpha, beta, and gamma radiation.
The Atom. Before defining ionizing radiation, it is useful to first describe an atom. Atoms are the basic building blocks of all elements. We have models of an atom that are supported by measurements. An atom consists of one nucleus, made of protons and neutrons, and many smaller particles called electrons. The electrons normally circle the nucleus much like the planets or comets circle the sun. The number of protons in the atom's nucleus determines which element it is. For example, an atom with one proton is hydrogen and an atom with 27 protons is cobalt. Each proton has a positive charge, and positive charges try to push away from one another. The neutrons neutralize this action and act as a kind of glue that holds the protons together in the nucleus. The number of protons in an atom of a particular element is always the same, but the number of neutrons may vary. Neutrons add to the weight of the atom, so an atom of cobalt that has 27 protons and 32 neutrons is called cobalt-59 because 27 plus 32 equals 59. If one more neutron were added to this atom, it would be called cobalt-60. Cobalt-59 and cobalt-60 are isotopes of cobalt. Isotopes are forms of the same element, but differ in the number of neutrons within the nucleus. Since cobalt-60 is radioactive, it is called a radionuclide. All isotopes of an element, even those that are radioactive, react chemically in the same way. Atoms tend to combine with other atoms to form molecules (for example, hydrogen and oxygen combine to form water). Radioactive atoms that become part of a molecule do not affect the way the molecule behaves in chemical reactions or inside your body.
What Ionizing Radiation Is. Ionizing radiation is energy that is carried by several types of particles and rays given off by radioactive material, x ray machines, and fuel elements in nuclear reactors. Ionizing radiation includes alpha particles, beta particles, x rays, and gamma rays. Alpha and beta particles are essentially small fast moving pieces of atoms. X rays and gamma rays are types of electromagnetic radiation. These radiation particles and rays carry enough energy that they can knock out electrons from molecules, such as water, protein, and DNA, with which they interact. This process is called ionization, which is why it is named "ionizing radiation." We cannot sense ionizing radiation, so we must use special instruments to learn whether we are being exposed to it and to measure the level of radiation exposure. The other types of electromagnetic radiation include radiowaves microwaves, ultrasound, infrared radiation, visible light, and ultraviolet light. These types of radiation do not carry enough energy to cause ionization and are called non-ionizing radiation. This profile will only discuss ionizing radiation.
What Ionizing Radiation Is Not. Ionizing radiation is not a substance like salt, air, water, or a hazardous chemical that we can eat, breathe, or drink or that can soak through our skin. However, many substances can become contaminated with radioactive material, and people can be exposed to ionizing radiation from these radioactive contaminants.
How Does an Atom Become Radioactive? An atom is either stable (not radioactive) or unstable (radioactive). The ratio of neutrons to protons within the nucleus determines whether an atom is stable. If there are too many or too few neutrons, the nucleus is unstable, and the atom is said to be radioactive. There are several ways an atom can become radioactive. An atom can be naturally radioactive, it can be made radioactive by natural processes in the environment, or it can be made radioactive by humans. Naturally occurring radioactive materials such as potassium-40 and uranium-238 have existed since the earth was formed. Other naturally occurring radioactive materials such as carbon-14 and hydrogen-3 (tritium) are formed when radiation from the sun and stars bombards the earth's atmosphere. The elements heavier than lead are naturally radioactive because they were originally formed with too many neutrons. Human industry creates radioactive materials by one of two different processes. In the first process, a uranium or a plutonium atom captures a neutron and splits (undergoes nuclear fission) into two radioactive fission fragments plus two or three neutrons. In a nuclear reactor, one of these "fission neutrons" is captured by another uranium atom, and the fission process is repeated. In the second process, stable atoms are bombarded either by neutrons or by protons that are given a lot of energy in a machine called an accelerator. The stable atoms capture these bombarding particles and become radioactive. For example, stable cobalt-59, found in the steel surrounding a nuclear reactor, is hit by neutrons coming from the reactor and can become radioactive cobalt-60. Any material that contains radioactive atoms is radioactive material.
How Does a Radioactive Atom Give off Ionizing Radiation? Because a radioactive atom is unstable, at some time in the future, it will transform into another element by changing the number of protons in the nucleus. This happens because one of several reactions takes place in the nucleus to stabilize the neutron-proton ratio. If the atom contains too many neutrons, a neutron changes into a proton and throws out a negative "beta" (pronounced bay' tah) particle. If the atom contains too many protons, normally a proton changes into a neutron and throws out a positive "beta" particle. Some atoms that are more massive than lead, such as radium, transform by emitting an "alpha" (pronounced al'- fah) particle. Any excess energy that is left can be released as "gamma" rays, which are the same as x rays. Other reactions are also possible, but the final result is to make a radioactive atom into a stable atom of a different element. For example, each atom of cobalt-60 is radioactive because it has too many neutrons. At some time in the future, one of its neutrons will change into a proton. As it changes, the atom gives off its radiation, which is a negative beta particle and two gamma rays. Because the atom now has 28 protons instead of 27, it has changed from cobalt into nickel. In this way, unstable atoms of radioactive cobalt-60 give off radiation as they transform into stable atoms of nickel-60.
How Long Can Radioactive Material Give Off Ionizing Radiation? Theoretically, it gives off ionizing radiation forever. Practically, however, after 10 half-lives, less than 0.1% of the original radioactivity will be left and the radioactive material will give off infinitesimally small amounts of ionizing radiation. The half-life is the time it takes one-half of the radioactive atoms to transform into another element, which may or may not also be radioactive. After one half-life, ½ of the radioactive atoms remain; after two half-lives, half of a half or 1/4 remain, then 1/8, 1/16, 1/32, 1/64, etc. The halflife can be as short as a fraction of a second or as long as many billions of years. Each type of radioactive atom, or radionuclide, has its own unique half-life. For example, technetium-99m and iodine-131, which are used in nuclear medicine, have 6-hour and 8-day half-lives, respectively. The naturally occurring radionuclide, uranium-235, which is used in nuclear reactors, has a half-life of 700 million years. Naturally occurring potassium- 40, which is present in the body, has a half-life of 13 billion years and undergoes about 266,000 radioactive transformations per minute in the body. Thus, technetium-99m will remain radioactive for 60 hours, and iodine-131 will remain radioactive for 3 months. On the other hand, long-lived naturally occurring uranium and potassium will remain, practically speaking, radioactive forever.
What Are the Three Types of Radiation? The three main types of ionizing radiation are called alpha, beta, and gamma radiation. These are named for letters of the Greek alphabet, and they are often symbolized using the Greek letters alpha (α), beta (β), and gamma (γ).
Alpha Radiation (or Alpha Particles). This type of radiation can be called either alpha radiation or alpha particles. Alpha radiation is a particle, consisting of two protons and two neutrons, that travels very fast and thus has a good deal of kinetic energy or energy of motion. The two protons and neutrons make an alpha particle identical to a helium atom, but without the electrons. Although it is much too small to be seen with the best microscope, it is large compared to a beta particle. The protons give it a large positive charge that pulls hard at the electrons of other atoms it passes near. When the alpha particle passes near an atom, it excites its electrons and can pull an electron from the atom, which is the process of ionization. Each time the alpha particle pulls an electron off from an atom in its path, the process of ionization occurs. With each ionization, the alpha particle loses some energy and slows down. It will finally take two electrons from other atoms at the end of its path and become a complete helium atom. This helium has no effect on the body. Because of their large mass and large charge, alpha particles ionize tissue very strongly. If the alpha particle is from radioactive material that is outside the body, it will lose all its energy before getting through the outer (dead) layer of your skin. This means that you can only be exposed to alpha radiation if you take radioactive material that produces alpha radiation into your body (for example, if you breathe it in or swallow it in food or drink). Once inside the body, this radioactive material can be mixed in the contents of the stomach and intestines, then absorbed into the blood, incorporated into a molecule, and finally deposited into living tissue such as the bone matrix. The alpha particles from this radioactive material can cause damage to this tissue.
Radiation (or Beta Particles). This type of radiation can be called either beta radiation or beta particles. Beta particles are high-energy electrons that some radioactive materials emit when they transform. Beta particles are made in one of two ways, depending on the radioactive material that produces them. As a result, they will have either a positive charge or a negative charge. Most beta particles are negatively charged. They are much lighter and much more penetrating than alpha particles. Their penetrating power depends on their energy. Some, such as those from tritium, have very little energy, and can't pass through the outer layer of dead skin. Most have enough energy to pass through the dead outer layer of a person's skin and irradiate the live tissue underneath. You can also be exposed to beta radiation from within if the betaemitting radionuclide is taken into the body. A beta particle loses its energy by exciting and ionizing atoms along its path. When all of its kinetic energy is spent, a negative beta particle (negatron) becomes an ordinary electron and has no more effect on the body. A positive beta particle (positron) collides with a nearby negative electron, and this electronpositron pair turns into a pair of gamma rays called annihilation radiation, which can interact with other molecules in the body.
Gamma Radiation (or Gamma Rays). This type of radiation can be called either gamma radiation or gamma rays. Unlike alpha and beta radiation, gamma radiation is not a particle, but is a ray. It is a type of light you cannot see, much like radio waves, infrared light, ultraviolet light, and x rays. When a radioactive atom transforms by giving off an alpha or a beta particle, it may also give off one or more gamma rays to release any excess energy. Gamma rays are bundles of energy that have no charge or mass. This allows them to travel very long distances through air, body tissue, and other materials. They travel so much farther than either alpha or beta radiation that the source of the gamma rays doesn't have to be inside the body or near the skin. The gamma ray source can be relatively far away, like the radioactive materials in nearby construction materials, soil, and asphalt. A gamma ray may pass through the body without hitting anything, or it may hit an atom and give that atom all or part of its energy. This normally knocks an electron out of the atom (and ionizes the atom). This electron then uses the energy it received from the gamma ray to ionize other atoms by knocking electrons out of them as well. Since a gamma ray is pure energy, once it loses all its energy it no longer exists.
How does radioactive material enter and spread through the environment?
Radioactive material can be released to the air as particles or gases as a result of natural forces and from human industrial, medical, and scientific activities. Everyone, with no exception, is exposed to ionizing radiation that comes from natural sources, such as cosmic radiation from space and terrestrial radiation from radioactive materials in the ground. Ionizing radiation can also come from industrially produced radioactive materials (such as iridium-192); nuclear medicine (such as thyroid cancer treatment with iodine-131 and thyroid scans using iodine-125, or bone scans using technetium- 99m); biological and medical research using carbon-14, tritium, and phosphorus-32; the nuclear fuel cycle (producing fission products such as cesium-137 and activation products such as cobalt- 60); and production and testing of nuclear weapons. Radioactive material released into the air is carried by the wind and is spread by mixing with air. It is diluted in the atmosphere and can remain there for a long time. When the wind blows across land contaminated with radioactive materials, radioactive particles can be stirred up and returned to the atmosphere. Radioactive material on the ground can be incorporated into plants and animals, which may later be eaten by people.
Water can contain man-made and naturally occurring radioactive materials that it dissolves from the soil it passes over or through. Rain and snow also wash man-made and naturally occurring radioactive material out of the air. Radioactive material may be added to water through planned or accidental releases of liquid radioactive material from sources such as hospitals, research universities, manufacturing plants, or nuclear facilities. Radioactive material can also reach surface waters when airborne radioactive materials settle to the earth or are brought down by rain or snow, and when soil containing radioactive material is washed away into a river or lake. The movement of liquid radioactive material is limited by the size of the bodies of water into which the radioactive materials have drained. Like silt, some radioactive material may settle along the banks or in the bottoms of ponds and rivers. In public health and ecological contexts, it is sometimes important to distinguish between dissolved radioactivity and radioactivity bound to suspended or settled solid particles. Radioactive material may also concentrate in aquatic animals and plants. Eventually, radioactive material in liquid runoff that goes into rivers and streams may reach the oceans (there are approximately one million radioactive transformations per minute of the naturally occurring radioactive potassium in one cubic meter of ocean water).
Radioactive material moves very slowly in soil compared to its speed of movement in air and water. Radioactive material will often stick to the surface of the soil. The organic material in soils can bind radioactive material, which slows its movement through the environment. If crops are watered with water containing radioactive material, the radioactive material may be taken up through the roots of the plant or may contaminate the outside of the plant. The plants may then be eaten by both animals and people. Radioactive materials that occur naturally in the soil (uranium, radium, thorium, potassium, tritium, and others) are also taken up by plants, and become available for intake by animals and people.
How might I be exposed to ionizing radiation?
The earth is continually irradiated with low levels of ionizing radiation, so all animals, plants, and other living creatures are exposed to small amounts of ionizing radiation from several sources every day.
Figure 1
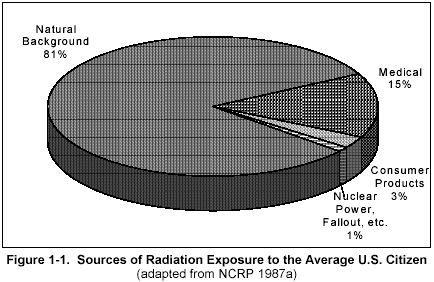
(click on image for a larger view)
Figure 1 shows that most of your radiation dose comes naturally from the environment. Smaller portions come from medicine, consumer products and other sources.
Figure 2
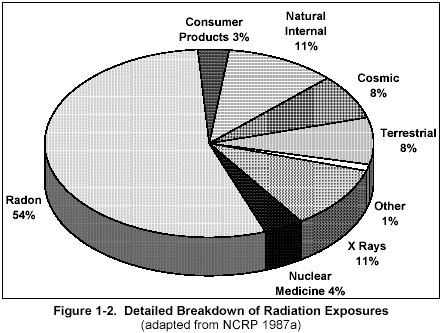
(click on image for a larger view)
Figure 2 is another breakdown of the sources of radiation dose to the average American. The natural background levels (82%) shown in Figure 1-1 include the radon, terrestrial, cosmic, and natural internal sources shown in Figure 1-2. Most of your daily radiation dose is from radon (55%), which is found in all air. Higher levels are normally found indoors (especially in the basement).
Figure 3 shows that indoor levels of radon vary depending on where you live. Higher levels can be found in underground areas, such as mines.
Figure 3
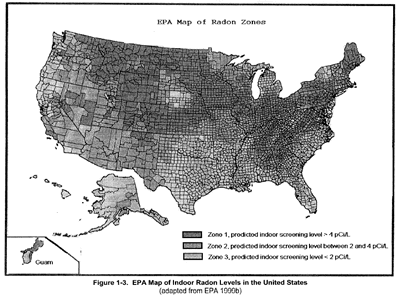
(click on image for a larger view)
You are always exposed to radiation from cosmic sources (mostly from outer space, some from the sun, 8%), terrestrial sources (rocks and soil, 8%), and natural internal sources (radioactive material normally inside your body, 10%). You may also be exposed to radiation from x ray exams (11%), nuclear medicine exams such as thyroid scans (4%), and consumer products including TV and smoke detectors (3%), as well as other sources.
Less than 1% of the total ionizing radiation dose to people living in the United States comes from their jobs, nuclear fallout, the nuclear fuel cycle, or other exposures. However, people in some types of jobs may have higher doses (pilots and flight attendants, astronauts, industrial and nuclear power plant workers, x ray personnel, medical personnel, etc.). Some groups of people have been exposed to higher-than- normal levels of ionizing radiation from weapons testing, and some individuals from accidents at nuclear facilities or in industry.
Not everyone will be exposed to every source or the same percentage of radiation shown in Figure 2. Since the percentages shown in Figure 2 are averages, half of the population will receive greater doses and half will receive smaller doses from the several sources shown in the figure. For example, if you are not regularly x rayed, you may receive less total radiation dose than what is shown. However, if you live in a town or city at a high altitude, you may receive a greater radiation dose from outer space cosmic rays than someone who lives in a town or city near the ocean at sea-level. Table 1 shows you that where you live and what you do determines how much ionizing radiation you will receive.
Table 1-1. Approximate Doses of Ionizing Radiation to Individuals
| Activity |
Approximate doses of radiation received |
Comments |
| Average American exposure to ionizing radiation |
| Total yearly dose |
360 mrem/yr
(3.6 mSv/yr) |
|
| From natural sources |
300 mrem/yr
(3.0 mSv/yr) |
|
| From man-made sources |
60 mrem/yr
(0.6 mSv/yr) |
|
| From nuclear power |
Less than 1 mrem/yr
(<0.01 mSv/yr) |
|
| Approximate doses of ionizing radiation (cosmic + terrestrial) for different locations |
| Kerala, India, resident |
1300 mrem/yr
(13 mSv/yr) |
Concentrated radioactive material in
the soil |
| Colorado state resident |
179 mrem/yr
(1.79 mSv/yr) |
High altitude above sea level |
| Boston, Massachusetts, resident |
100 mrem/yr
(1 mSv/yr) |
|
| Louisiana state resident |
92 mrem/yr
(0.92 mSv/yr) |
Low altitude above sea level |
| Approximate doses of ionizing radiation above background radiation and some activities |
| Anyone near a patient released after a nuclear medicine
test. |
Less than 500 mrem/patient
(5 mSv/patient) |
Guidance for medical facilities. Quantity
depends on the quantity of radioactive material. |
| A person who works inside a nuclear power plant |
< 300 mrem/yr
(<3 mSv/yr) |
|
| A person who gets a full set of dental x rays |
40 mrem
(0.4 mSv) |
|
| A flight attendant flying from New York to Los Angeles |
5 mrem/flight
(0.05 mSv/flight) |
|
| Watching a color TV set |
2-3 mrem/yr
(0.02-0.03 mSv/yr) |
|
| A person who lives directly outside of a nuclear power
plant |
1 mrem/yr
(0.01 mSv/yr) |
|
| A person who lives in a multi-storied apartment building |
~1 mrem/yr for each 5 stories above
the ground floor
(<0.01 mSv/yr) |
Difference between Los Angeles and
Denver = 87 mrem/5000 feet = 2 mrem/100 feet = 1 mrem/5
stories |
| A person who watches a truck carrying nuclear waste
pass by |
Less than 0.1 mrem/truck
(0.001 mSv) |
|
"Dose" is a broad term that is often used to mean either absorbed dose, or dose equivalent, depending on the context. The absorbed dose is measured in both a traditional unit called a rad and an International System (S.I.) unit called a gray (Gy). Both grays and rads are physical units (1 Gy = 100 rad) that measure the concentration of absorbed energy. The absorbed dose is the amount of energy absorbed per kilogram of absorber. Physical doses from different radiations are not biologically equivalent. For this reason, a unit called the dose equivalent, which considers both the physical dose and the radiation type, is used in radiation safety dosimetry. The unit of dose equivalent is called the rem in traditional units and the sievert (Sv) in S.I. units (1 rem = 0.01 Sv). For beta and gamma radiation, 1 rad = 1 rem (1 gray = 1 sievert). For alpha radiation, however, 1 rad = 20 rem (1 gray = 20 sievert). Small radiation doses can be expressed using small dose units such as the millirem (mrem) and the millisievert (mSv), where 1 mrem = 0.001 rem and 1 mSv = 0.001 Sv.
The average annual dose to a person in the United States is about 360 mrem (3.6 mSv). An individual's exact dose depends on several factors, such as the natural background where the person lives, and the person's medical history and occupational experience with sources of radiation.
How can ionizing radiation enter and leave my body?
Ionizing radiation exposure can occur from a radiation source outside of the body. Exposure can also occur as a result of taking radioactive material into your body. The answer to the question of how you can be exposed to ionizing radiation can be broken into two parts. The first paragraph below describes ionizing radiation that comes from a source outside your body and some distance away (external radiation). The second paragraph describes ionizing radiation that comes from a source inside your body (internal radiation).
External radiation comes from natural and manmade sources of ionizing radiation that are outside your body. Part of the natural radiation is cosmic radiation from space. The rest is given off by radioactive materials in the soil and building materials that are around you. As a result of human activities, higher levels of natural radioactive material are left in products or on the land. Examples of such activities are manufacturing fertilizer, burning coal in power plants, and mining and purifying uranium. Ionizing radiation from human activities adds to your external radiation exposure. Some of this radiation is given off by x ray machines, televisions, radioactive sources used in industry, and patients who have had recent nuclear medicine tests and therapy. The rest is given off by man-made radioactive materials in consumer products, industrial equipment, atom bomb fallout, and to a smaller extent by hospital waste and nuclear reactors. Gamma rays are the main type of ionizing radiation that are of concern when you are exposed to external sources of ionizing radiation. Gamma rays (like x rays) are special bundles of light energy that you cannot see, feel, or smell. Gamma rays from natural and man-made sources pass through your body just like x rays do, at the speed of light. Gamma rays may pass directly through your body without hitting anything. When one gamma ray hits a cell, it leaves a small bit of energy behind that can cause damage. Other types of ionizing radiation, like alpha and beta particles, hit your body but normally do not have enough energy to get inside to harm you. Your external radiation dose depends on the amount of energy that ionizing radiation gives to your body as it passes through. Exposure to external radiation does not make you radioactive. The average yearly dose from external radiation in the United States is about 100 mrem per person (1 mSv/person).
Internal radiation is ionizing radiation that natural and man-made radioactive materials give off while they are inside your body. You take radioactive materials into your body every day since they are in the air you breathe, the food you eat, and the water you drink. Examples of natural radioactive materials that enter, reside in, and leave your body every day include potassium-40, carbon-14, radium, and radon. Man-made radioactive materials also get into your body from the decreasing amounts of fallout from past nuclear weapons testing. Sometimes, natural conditions or industrial activities concentrate radioactive materials. If you are exposed to these, you will take in more radioactive material. Low amounts of material that act as sources of ionizing radiation may also be put into your body for medical purposes to test for or treat some types of disease, such as cancer.
Scientists and clinicians have made sure that the benefits of exposing you to ionizing radiation far outweigh any bad health effects you may get from the ionizing radiation by itself. (Medical tests use small amounts of radiation or radioactive material, but some radiotherapy uses large doses that are beneficial to the patient.) Hospitals, coal-fired electricity generating plants, and nuclear reactors release radioactive materials in ways that keep your dose low. Radioactive materials build up in your body if you take them in faster than they leave in urine and feces and by radioactive transformation. If the internally deposited radioisotope is short lived and decays before the body eliminates it, then, of course, it will disappear faster from the body than by biological elimination alone. Thus, retention or elimination of internally deposited radioisotopes is measured by the effective half-life, which considers the combined effect of biological elimination and radioactive decay.
Internally deposited radioisotopes may emit gamma rays, beta particles, or alpha particles, depending on the isotope. Many gamma rays escape your body without hitting anything. When a gamma ray does hit a cell, it transfers energy to the cell. When all their energy is transferred, they vanish. Alpha and beta particles travel short distances, giving energy to cells they hit. They lose energy and quickly come to a stop. Their energy is totally absorbed inside your body. When alpha particles come to a stop, they become helium that you breathe out later. When beta particles come to a stop, they become electrons and attach to atoms near them. Your internal dose is a measure of the energy deposited by all the ionizing radiation that is produced inside your body. The average yearly dose in the United States from internal radiation is about 260 mrem per person (2.6 mSv/person).
How can ionizing radiation affect my health?
How radiation affects your health depends on the size of the radiation dose. Scientists have been studying the effects of ionizing radiation in humans and laboratory animals for many years. Studies so far have not shown that the low dose of ionizing radiation we are exposed to every day causes us any harm. We do know that exposure to massive amounts of ionizing radiation can cause great harm, so it is wise to not be exposed to any more ionizing radiation than necessary.
Overexposure to high amounts of ionizing radiation can lead to effects like skin burns, hair loss, birth defects, cancer, mental retardation (a complex central nervous system functional abnorm ality), and death. The dose determines whether an effect will be seen and its severity. For some effects such as skin burns, hair loss, sterility, nausea, and cataracts, there is a certain minimum dose (the threshold dose) that must be exceeded to cause the effect. Increasing the size of the dose after the threshold is exceeded makes the effect more severe. Psychological stress has been documented in large populations exposed to small doses of radiation (Three Mile Island and Chernobyl). Neurological injury (CNS syndrome) resulting in compromised mental function has also been documented in individuals exposed to several thousand rads of ionizing radiation.
Ionizing radiation is called a carcinogen because it may also increase your chance of getting cancer.
Increasing the size of the dose increases your chance of getting cancer. Scientists base radiation safety standards on the assumption that any radiation dose, no matter how small, carries with it a corresponding probability of causing a cancer. This is called a "zero threshold" dose response relationship. Cancers that are actually caused by radiation are completely indistinguishable from those from other causes, so we can never be certain whether any individual cancer was not caused by radiation. To determine how likely it is that a certain dose of radiation will cause cancer, scientists measure the radiation dose to a group of exposed people, like the Japanese atomic bomb survivors. Then they compare the frequency of cancers (the observation period for cancer extends over decades) in this exposed group with a similar group of people who were not exposed. They also look at factors like age, sex, and time since the exposure ended. Finally, they calculate risk factors for various cancer types. Using these factors, it is possible to estimate the chance of getting cancer from a dose of radiation. Even though they assume a zero threshold, researchers have not actually seen an increase in cancer frequency for people in the exposed Japanese group who had a radiation dose below 20 rad (0.2 Gy). No increase in any type of leukemia has been found in people whose radiation dose was below 10–40 rad (0.1–0.4 Gy).
The effects of internally deposited radioactive material are similar to those of external radiation. The effects depend on the size of the dose and factors like your sex and age when you were exposed. The radiation absorbed dose, in turn, depends on the radioactive material, the amount of activity, the type and energy of the radiation, the effective half-life of the radioactive material, its chemical form, how it was taken into your body, and how quickly it leaves your body.
Many people are exposed to radiation and radioactive materials used in medical testing and therapy. Radiation treatments for medical reasons carry the same risk as radiation from other sources. As with any medical treatment, the potential health benefits should be balanced against the potential harmful health effects.
One way to better understand the effects of radiation is to study its effects on test animals. Without laboratory animals, scientists would lose a basic method to get information needed to make informed decisions to protect public health. Scientists have the responsibility to treat research animals with care and compassion. Laws today protect the welfare of research animals, and scientists must comply with strict animal care guidelines.
How can ionizing radiation affect children?
This section discusses potential health effects from exposures during the period from conception to maturity at 18 years of age in humans. Potential effects on children resulting from exposures of the parents are also considered.
Like adults, children are exposed to small background amounts of ionizing radiation that comes from the soil around where they live, in the food and water that they eat and drink, in the air that they breathe, and from sources that reach earth from space. How much background radiation you receive depends on where you live. Some places naturally have more than others. There are no reports that say exposure to background levels of ionizing radiation causes health effects in children or adults.
If a pregnant woman is exposed to high levels of ionizing radiation, it is possible that her child may be born with some brain abnormalities. There is an 8-week period during early pregnancy when an unborn child is especially sensitive to the effects of higher than normal levels of ionizing radiation. As the levels of ionizing radiation increase, so does the chance of brain abnormalities. These abnormalities may eventually result in small head size, decreased intelligence as measured by Intelligence Quotient (IQ) tests, and other defects. These effects are not reversible.
A child will be exposed to small amounts of radiation from the environment all during prenatal development and throughout its life. There are no reports that say children suffer health effects from normal amounts of background radiation. If children are exposed to higher than background levels of ionizing radiation, they are likely to have the same possible health effects as adults exposed to similar levels.
How can families reduce the risk of exposure to ionizing radiation?
If your doctor finds that you have been exposed to significant amounts of ionizing radiation, ask whether your children might also be exposed. Your doctor might need to ask your state health department to investigate.
The best way to reduce your risk of exposure to higher than background amounts of radiation is to not let yourself be exposed at all. However, this is not always possible or sensible. A common way to be exposed to ionizing radiation is by receiving an x ray, but a few x rays every year will not hurt you. When you or your children receive an x ray, be sure to correctly wear any protective garments that are provided. The technician will make sure that only the area that needs to be x rayed will be exposed to the x ray beam.
It may be necessary to inject you with a chemical that has some amount of radioactive material in it to help a doctor diagnose or treat a disease. Many studies have shown that these drugs, used correctly, will not harm you. Be sure to follow the doctor's directions after you have been treated with these drugs.
Many places make or use various types of radioactive material or ionizing radiation for medical or research purposes. If you visit one of these facilities, be sure to follow all of the recommended safety precautions. Do not go into unauthorized areas. You may be asked to wear a special device on your shirt that records the amount of ionizing radiation you are exposed to while in the facility. This is a safety precaution. Do not put it in your pocket or let someone else wear it.
Is there a medical test to determine whether I have been exposed to ionizing radiation?
There are no easy or accurate medical tests to determine whether you have been exposed to low doses of ionizing radiation, but tests are available for determining whether you have been exposed to radioactive material.
Tests for Recent Exposure to Ionizing Radiation. A great degree of overexposure is necessary to cause the clinical signs or symptoms of radiation exposure. In the absence of clinical signs or symptoms there are two kinds of tests scientists use to see if you have been overexposed to ionizing radiation; they look for changes in blood cell counts and changes in your chromosomes. If you are exposed to no more than 10 rad (0.1 Gy) of ionizing radiation, there are no detectable changes in blood cell counts. The most sensitive measure of radiation exposure involves a study of your chromosomes. This is a special test for doses that are too low to produce clinically observable signs or symptoms; this test may be useful for doses greater than about 3 times the maximum annual permissible dose for radiation workers. Changes in the white blood cell count may be seen in people whose doses exceeded about 5 times the occupational maximum permissible annual dose. Radiation doses at or above these levels can be reliably estimated using these two special tests.
Tests for Radioactive Material Inside Your Body. Scientists can also examine your blood, feces, saliva, urine, and even your entire body to see if measurable amounts of radioactive material are being excreted from your body. Different tests are used for different types of radioactive material. Several types of instruments are available to look for each type of radiation. These instruments are not available at your doctor's office. They are normally large, heavy, and available only in laboratories. Equipment usually consists of a "detector," electrical cables, and a "processor." The detector contains material sensitive to one or more types of radiation, so the detector is chosen based on the type of radiation to be measured. Alpha, beta, and gamma radiation have different energies that depend upon the radioactive isotope from which they come. By determining the type and energy of the radiation, scientists can tell which radioisotope is on your skin or inside your body.
What recommendations has the federal
government made to protect human health?
Recommendations and regulations are periodically updated as more information becomes available. For the most current information, check with the federal or state agency or organization that provides it.
The current federal and state regulations limit radiation workers' doses to 0.05 Sv/year (5 rem/year). The limit for the unborn child of a female radiation worker is 0.005 Sv (0.5 rem) per 9- month gestation period. For the general public, the limit is 0.001 Sv/year (0.1 rem/year), with provisions for a limit of 0.005 Sv/year (0.5 rem/year) under special circumstances. The public dose limit is set at least 10 times lower than the occupational limit to give the public an extra margin of safety. A factor of 10 is also used for public protection in other industries.
We have seen health effects from very high doses of ionizing radiation, but not at normal everyday levels. To be cautious, scientists and regulating agencies assume that there could be some harmful effects at any dose, no matter how small. Because ionizing radiation has the potential to cause harmful health effects in overexposed people, regulations and guidelines have been established for ionizing radiation by state, national, and international agencies. The basic philosophy of radiation safety is to allow only a reasonable risk of harm using the concept of "as low as reasonably achievable" (ALARA). Some regulations and recommendations for ionizing radiation include the following:
Radiation protection standards for radiation workers and members of the public are recommended by the International Commission on Radiological Protection (ICRP) and the National Council on Radiation Protection and Measurements (NCRP). These standards are not regulations, but they provide the scientific basis for the making of regulations by federal agencies. The ICRP and NCRP are authoritative bodies that analyze current scientific and epidemiological data and make recommendations to government and nongovernment organizations that set standards. ICRP and NCRP do not issue standards themselves.
Federal agencies, such as the EPA, the Nuclear Regulatory Commission (NRC), and the Department of Energy (DOE), as well as individual states are responsible for making federal and state regulations about exposure to ionizing radiation. The NRC regulates nuclear power plant operations and regulates the use of radioactive material in research and medical applications. The DOE has issued employee dose limits for its facilities.
The EPA is responsible for federal radiation protection guidance for environmental radiation standards and regulations to implement specific statutory requirements, such as the Safe Drinking Water Act and the Clean Air Act. Natural background radiation, of course, cannot be regulated but EPA recommends that the concentration of indoor radon not exceed 4 picocuries per liter (4 pCi/L) of air. EPA's National Emission Standards for Hazardous Air Pollutants (NESHAPs) contain regulations that limit the dose from radionuclides released to the air to 0.1 mSv/year (10 mrem/year). The EPA sets limits on the maximum acceptable concentration of radionuclides in public drinking water supplies. Based on the Safe Drinking Water Act, the EPA has issued drinking water standards for radionuclides, which include dose limits of 0.04 mSv/year (4 mrem/year) for man-made sources of beta and gamma emitters. EPA also sets limits on several alpha emitters in drinking water, such as radium and radon.
The NRC regulations apply to all types of ionizing radiation that are emitted from special nuclear material (such as nuclear reactor fuel) and from byproduct material (materials made radioactive in the use of special nuclear material), and from source material (material from which nuclear fuel is made).The NRC sets limits on the total dose of ionizing radiation above background from these sources. It also sets limits for the amounts and concentrations of radioactive material that will give these doses if taken into the body. These are called Annual Limits on Intake (ALI) and derived air concentrations (DAC).
The NRC has also issued a standard for cleaning up sites contaminated with radioactive materials. It requires that the radiation dose to the public from these sites will not be more than 0.25 mSv per year (25 mrem per year).
Radiation doses from procedures used by licensed physicians in diagnosis and treatment of disease is not limited by regulations. However, physicians and medical technicians must be specially trained and licensed to use radiation-producing machines and licensed to use radioisotopes for these purposes. They are required to limit exposures to the members of the public who are inside their facilities to 100 mrem per year, which is the same level as required by the NRC. Also, patients with radioactive materials inside their bodies from the treatment are kept until it is likely that they will not expose anyone around them to more than 0.5 mSv (500 mrem) from that radioactive material.
States also regulate radioactive materials and other sources of radiation that are not regulated by the NRC. These include sources of natural radioactivity, such as radium, and radiationproducing machines, such as x ray machines and radioactive material produced by particle accelerators.
References
Agency for Toxic Substances and Disease Registry (ATSDR). 1999. Toxicological profile for ionizing radiation. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
Where can I get more information?
If you have questions or concerns, please contact your community or state health or environmental quality department or:
For more information, contact:
Agency for Toxic Substances and Disease Registry
Division of Toxicology and Human Health Sciences
4770 Buford Highway
Chamblee, GA 30341-3717
Phone: 1-800-CDC-INFO 888-232-6348 (TTY)
Email: Contact CDC-INFO
ATSDR can also tell you the location of occupational and environmental health clinics. These clinics specialize in recognizing, evaluating, and treating illnesses resulting from exposure to hazardous substances.